Immunomodulatory Effects of Dietary Whey Proteins | Equine Clinical Research
Journal of Dairy Research (1995) 62 359-368
Reprinted with permission of Cambridge University Press
©1995
Immunomodulatory effects of dietary whey proteins in mice
By CHUN W. WONG AND DENNIS L. WATSON
CSIRO Division of Animal Health, Armidale, NSW 2350, Australia
SUMMARY. Studies on the immunomodulatory properties of dietary whey proteins in mice are reported. Ingestion of bovine milk whey proteins, either as a supplement in an adequately balanced commercial diet or as the only protein source in a balanced diet, consistently enhanced secondary humoral antibody responses following systemic immunization with ovalbumin, when compared with other protein sources such as soyabean protein isolate and ovine colostral whey proteins. After 5-8 weeks of feeding, dietary milk whey proteins enhanced cell-mediated immune responses as revealed by footpad delayed type hyper-sensitivity responses, and coneanavalin A-induced spleen cell proliferative responses. To monitor nutritional effects of milk whey proteins, live weight, leucocyte counts and clinical changes of diet-fed mice were examined. The present results confirm other previous results that dietary bovine milk whey proteins have immunoenhancing properties in mice and these properties are unlikely to be related solely to the nutritional effects.
Whey contains a multitude of proteins that remain soluble after precipitation of caseins during the manufacture of cheese (Eigel et al. 1984). There are major components such as ß-lactoglobulin, a-lactalbumin, serum albumin and immuno-globulins, and minor components such as lactoferrin, lactoperoxidase (EC 1. 11. 1.7) and various growth factors. Many of these components possess immunobiological properties (Ogra & Ogra, 1978; Juto, 1985; Stoeck et al. 1989; Mincheva-Nilsson et al. 1990; Watson. 1990; Barta et al. 1991). It is evident that peptides and the amino acids incorperated in them can influence the immune response in different ways, and minor changes in the dietary amino acid profile may modulate the immune response without significant impact on nutritional status (Belokrylov et al. 1992). Therefore, it is possible that, unique amino acid groups or peptides derived from whey proteins after ingestion may have significant immunomodulatory activities in vivo.
Recent evidence suggests that a diet based on whey protein could enhance the IgM plaque-forming cell response in mouse spleen and prevent colon tumour growth in mice when compared with diets containing other protein sources (Bounous et al. 1988a, b). The prophylactic potential of whey proteins against initiation of colon tumours was further supported by a more recent study using a rat model (McIntosh, 1993). In addition, results from a preliminary clinical trial led Bounous et al. (1993) to propose that dietary whey proteins may have beneficial effects in human immunodeficiency virus (HIV)-infected patients. In view of these recent studies, a better understanding of the immunological properties of whey proteins and their underlying mechanisms would be of value in assessing their potential clinical or pharmaceutical applications. In the present study we have investigated the effects of dietary bovine milk whey protein concentrate on both humoral and cell-mediated immune responses in mice.
MATERIALS AND METHODS
Mice
Female BALB/c mice, 8-10 weeks old, were obtained from the CSIRO Division of Biomolecular Engineering (North Ryde, NSW 2113).
Diets
Several experimental approaches were used to evaluate the effects of dietary whey proteins on immune responses in mice. In Expt I, mice (seven per group) were fed on a commercial formulated mouse diet containing 230 g crude protein/kg (Fielders Agricultural Products, Tamworth, NSW 2340). Milk whey (CSIRO Dairy Research Laboratory, Highett, VIC 3190; 0.06 g protein/l) or >INFASOY= (Wyeth Pharmaceuticals Ltd, NSW 2150), a milk-free soyabean protein isolate (SPI) formula (0.06 g protein/l after reconstitution) or water alone was offered ad lib. in their drinking bottles.
In Expt II, mice (seven per group) were fed on the commercial diet. Milk whey as used in Expt I, ovine colostral whey (CSIRO Pastoral Research Laboratory Armidale, NSW 2350; 6 g protein/1) or water alone was offered ad lib. in their drinking bottles.
In Expt III. mice (a total of 36 per diet and 6 in each cage) were fed on the commercial diet for 2-3 weeks prior to being transferred to the following dietary treatments. A defined protein-free formula diet (ICN Biochemicals. Inc., Costa Mesa, CA 92626, USA) supplemented with either whey protein concentrate (WPC, CSIRO Dairy Research Laboratory: 200 g/kg diet) or SPI (ICN Biochemicals. 200 g/kg diet) as the only protein source was offered to mice, and water was offered ad lib.
Immunization
The mice were immunized by an intraperitoneal injection of 20 mg ovalbumin (Sigma, St Louis, MO 63178, USA) in dextran sulphate (0.5 g/l) constituted in 1 ml of sterile saline, and were given booster immunizations 2 weeks later. Immunization schedules are shown in the Results.
Experimental procedures
For Expts I and II, serum anti-ovalbumin levels were measured at weekly intervals. For Expt III, mice were selected at different time intervals for immunological measurements including footpad delayed-type hypersensitivity responses (3-6 mice per group), stimulated spleen cell responses (3-6 mice per group) and serum anti-ovalbumin antibody levels (all remaining mice, i.e. 11-36 per group). Diet consumption, body weight and leucocyte counts were measured and routine clinical observations carried out regularly throughout the experiments.
Antibody assay
Mice were bled from the retro-orbital sinus and serum was separated by centrifugation. An enzyme-linked immuno-sorbent assay (ELISA) was used to measure anti-ovalbumin levels in serum. To establish standards for the assay, five healthy mature female BALB/c mice were immunized intraperitoneally with 1 mg ovalbumin in 0.5 ml dextran sulphate twice at an interval of 10 d. The mice were then bled 7 d after the booster, and the sera pooled and used as the standard for the ELISA. Immulon Microtiter plates (Dynatech Laboratories Inc., VA 22021, USA) were coated overnight with 0.1 ml ovalbumin (10 mg/ml in 0.05 M?carbonate buffer, pH 9.6). After washing the plates with phosphate?buffered saline containing Tween 20 (0.5 g/l), optimal dilutions of serum were added, followed by optimal dilutions of goat anti?mouse IgG conjugated with alkaline phosphatase (Cappel Research Products, Durham, NC 27704, USA) and p-nitro-phenyl phosphate substrate (Sigma), with washing between steps. The reaction was terminated with 3 M-NaOH and the trays were read in a Titertek Multiskan MC ELISA reader (Flow Laboratories, NSW 2113) at 405 nm. A regression equation was used to convert absorbances to antibody units per ml:
log2(antibody units/ml) = (corrected absorbance (A405) + 0.341)/0.118.
Delayed-type hypersensitivity assay
Delayed-type hypersensitivity to sheep red blood cells was assessed by the footpad assay. Mice were immunized by subcutaneous injection of 1 x 108 sheep red blood cells in 250 µl saline. Six days later, the delayed-type hypersensitivity response was induced by injecting 1 x 108 sheep red blood cells in 25 µl saline into the right hind footpad and 25 µl saline into the left as a control (Liew, 1977). Footpad swelling was measured 24 h after challenge with the aid of dial calipers (Mitutoyo, Tokyo 160, Japan), and the results were expressed as increase in footpad thickness.
Spleen cell mitogen and antigen responses
Spleens were removed aseptically from mice. Spleen cell suspensions were prepared by teasing the organs apart with forceps, tamping them through a fine stainless steel wire mesh and collecting in RPMI 1640 medium (ICN Biochemicals) supplemented with heat-inactivated fetal calf serum (100 ml/l ICN Biochemicals). Only lymphoid cell suspensions with > 95% viability as determined by eosin dye exclusion tests were used in this assay. Flat bottomed 96-well microtitre plates (ICN Biochemicals Inc.) were used for culture. Into each well were dispensed 100 µl RPMI 1640 medium and 50 µl cell suspension (2 x 106 cells/ml). followed by 50 µl concanavalin A (1.95 µg/ml, Sigma), or lipopolysaccharides (15.6µg/ml. Escherichia coli serotype 055: B5. Sigma) or ovalbumin (75 µg/ml, Sigma). Control cultures received 50 µl medium only. All cultures were incubated at 37ºC in CO2-air (5:95 v/v) at > 95 % humidity for 3 d. Six hours prior to harvesting the cells on to glass fibre filter strips (ICN Biochemicals). 0.6 µCi [3H]thymidine (Amersham International, Amersham HP7 9NA, UK) in a 20 µl volume was added to each well. The samples were counted in a liquid scintillation ß counter.
Statistical analysis
Results were analysed using Student's t test, profile analyses or analyses of variance (ANOVA). When the treatment effect was significant by ANOVA, the significant difference between groups was determined by least significant difference (LSD) test.
RESULTS
Daily intake, live weights and leucocyte counts
Results for average daily fluid and feed intake, live weight and leucocyte counts of mice are shown in Table 1. No clinical abnormalities were observed in mice during the experiments.
In Expts I and II, milk whey proteins, SPI or ovine colostral whey was offered to mice as a supplement to a nutritionally balanced diet. Although the average daily fluid intake was significantly higher in the SPI group (P < 0.05) in Expt I, there was no significant difference in live weight. The leucocyte count was found to be lower (P < 0.05) in the SPI group than in other groups. In Expt II, the mice offered water had lower daily fluid intakes (P < 0.05). Again, no significant difference was observed for their body weight but the leucocyte count was found to be higher (P < 0.01) in the group given milk whey than in the other groups. However, the values of all leucocyte counts in both expts I and II correspond to published normal ranges (Schalm et al. 1986).
In Expt III, WPC or SPI was given as the sole protein source in the diets; there were no significant differences between treatment groups for feed intake or leucocyte counts.
Although the initial live weight was different (P < 0.05) in these two groups, the difference had disappeared by 2 weeks on the diets, before the first immunization was introduced.
| Table 1. Daily intakes, live weights, and leucocyte counts for mice in Experiments I, II and III | |||||||||||
| (Values are mean ± SEM) | |||||||||||
| Time on diet, weeks... | Fluid & Feed intake† | Live weight, g | Leucocytes (x 103)ml‡ | ||||||||
| 0 | 2 | 3 | 5 | 7 | 0 | 2 | 5 | 8 | |||
| Expt I | Milk whey (n=7) Soyabean protein isolate (n=7) Water (n=7) | 29.7 ± 0.6 44.7 ± 0.5* 24.2 ± 0.5 | 22.8 ± 0.7 21.6 ± 0.8 22.9 ± 0.4 | 24.0 ± 0.8 23.9 ± 0.8 24.1 ± 0.4 | ND ND ND | 25.0 ± 0.9 23.1 ± 0.5 24.7 ± 0.6 | ND ND ND | 9.9 ± 1.0 9.9 ± 1.0 9.9 ± 1.0 | ND ND ND | ND ND ND | 6.5 ± 0.6 4.9 ± 0.5* 6.8 ± 0.5 |
| Expt II | Milk whey (n=7) Ovine colostral whey (n=7) Water (n=7) | 31.7 ± 0.6 31.2 ± 0.5 23.6 ± 0.5* | 20.4 ± 0.7 20.7 ± 0.5 19.7 ± 0.8 | 21.6 ± 0.9 22.2 ± 0.7 22.3 ± 0.9 | ND ND ND | 20.7 ± 1.0 21.3 ± 0.6 21.5 ± 0.8 | ND ND ND | 7.0 ± 0.8 7.0 ± 0.8 7.0 ± 0.8 | ND ND ND | ND ND ND | 6.1 ± 0.3** 3.3 ± 0.8 4.3 ± 0.4 |
| Expt III | Whey protein concentrate (n=12-36) Soyabean protein isolate (n=11-36) | 2.5 ± 0.1 2.5 ± 0.1 | 17.1 ± 0.2 19.0 ± 0.3* | 21.1 ± 0.3 21.9 ± 0.3 | 21.7 ± 0.3 21.7 ± 0.4 | 21.7 ± 0.3 21.8 ± 0.3 | 23.3 ± 0.4 22.4 ± 0.4 | 5.3 ± 0.4 4.3 ± 0.2 | 7.4 ± 0.5 6.6 ± 0.3 | 6.0 ± 0.4 5.7 ± 0.4 | 4.9 ± 0.8 3.6 ± 0.9 |
| ND. Not determined † For Expts I and II, values are ml/head; for Expt III, g/head ‡ For Expts I and II, samples were taken from seven mice randomized into different diet groups afterwards. Values were significantly different from other values in the same column and experiment by ANOVA and LSD analyses: *P<0.05, **P<0.01. | |||||||||||
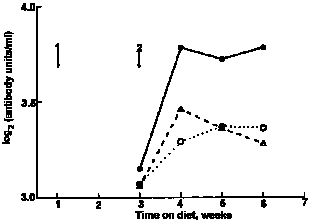 |
| Fig. 1. Mean serum anti-ovalbumin concentrations in mice offered milk whey; O, water or soyabean protein isolate in their bottles in Expt I. For details, see text. Arrows show timing of 1, primary and 2, secondary immunization with ovalbumin. |
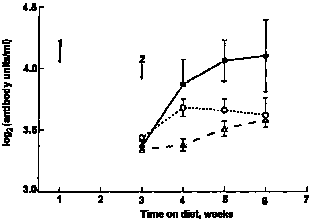 |
| Fig. 2. Serum anti-ovalbumin concentrations in mice offered, milk whey; O, water or ovine colostral whey in their drinking bottles in Expt II. For details, see text. Values are means with SEM indicated by vertical bars. Arrows show timing of 1, primary and 2, secondary immunization with ovalbumin. |
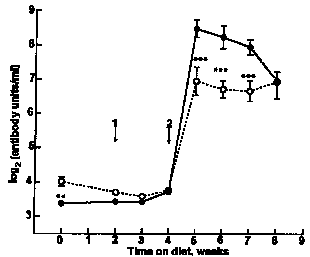 |
| Fig. 3. Serum anti-ovalbumin concentrations in mice offered, whey protein concentrate or O, soyabean protein isolate in Expt III. For details, see text. Values are means with SEM indicated by vertical bars. Arrows show timing of 1, primary and 2, secondary immunization with avalbumin. Values for the two treatments were significantly different by ANOVA and LSD analyses: **P<0.01, ***P<0.001. |
Antibody responses
In Expt I (Fig. 1), the secondary anti-ovalbumin response of mice ingesting milk whey was significantly higher than that of mice ingesting water or SPI (profile analysis P = 0.03). In Expt II (Fig. 2), mice that drank milk whey had higher mean serum anti-ovalbumin titres than did those that drank water or colostral whey (profile analysis P = 0.01). In Expt III (Fig. 3), peak serum antibody levels were higher than those recorded for Expts I and II. Although the mice were randomized at the beginning of this experiment before the immunization was introduced, a higher serum antibody level was observed in the SPI diet group. Whether this was related to their difference in body weight remains unclear. However, no such difference between two groups was found afterwards until the secondary immunization was introduced. Then, mice fed on the WPC diet again had significantly higher secondary anti-ovalbumin responses than did those fed on the SPI diet (P < 0.001).
Delayed type hypersensitivity responses
The footpad delayed-type hypersensitivity response to sheep red blood cells in mice has been widely used to assess T cell-mediated immune responses. In this study, the delayed-type hypersensitivity response of mice given WPC was found to be significantly higher than that of mice given SPI from week 5 of feeding to at least week 8 (Table 2).
Spleen cell responses
A dose-response experiment was performed to determine optimal concentrations of mitogen and antigen in the assay (Table 3). The splenocyte responses to lipopolysaccharides and ovalbumin did not differ statistically between the groups given SPI and WPC, but the response to concanavalin A, a T cell mitogen, at 8 weeks after feeding was found to be significantly higher in the mice given WPC (Table 4).
| Table 2. Delayed-type hypersensitivity responses of mice offered whey protein concentrate or soyabean protein isolate | |||
| (Values are increases in footpad thickness µm, expressed as means ± SEM for n = 3-6) | |||
| Time on diet, weeks | |||
| Diet | 5 | 6 | 8 |
| Whey protein concentrate | 874 ± 44 | 599 ± 33 | 985 ± 42 |
| Soyabean protein isolate | 591 ± 62 | 342 ± 58 | 812 ± 20 |
| Significance of difference, P < | 0.001 | 0.001 | 0.05 |
| Table 3. Effects of stimulant concentrations on spleen cell proliferative responses in BALB/c mice | ||
| Stimulants | Concentrations, µg/ml | [3H] thymidine uptake (mean of n = 2), cpm |
| Concanavalin A | 0.49 1.95† 7.80 31.25 | 39,070 53,040 6,020 2,650 |
| Lipopolysaccharide (Esch. coli 055: B5) | 3.90 15.60† 62.50 250.00 | 9,850 10,330 9,620 3,420 |
| Ovalbumin | 18.80 37.50 75.00† 150.00 | 1,190 2,030 4,240 2,480 |
| † Optimal concentrations used for studies on dietary supplementation (Table 4). | ||
| Table 4. Effects of feeding whey and soyabean proteins on stimulant-induced spleen cell proliferative responses from BALB/c mice | ||||
| (Values are means ± SEM for n=3-6) | ||||
| Stimulants | Time on diet, weeks | [3H] thymidine uptake, cpm | ||
| Whey protein concentrate | * | soyabean protein isolate | ||
| Concanavalin A (1.95 µg/ml) | 5 6 7 8 | 70,990 ± 8,830 39,130 ± 1,590 55,220 ± 6,220 136,880 ± 6,880 | 51,570 ± 5,550 45,830 ± 15,570 49,850 ± 8,320 84,390 ± 18,410 | |
| Lipopolysaccharide (Esch. coli 055: B5; (15.6 µg/ml) | 5 6 7 8 | 1,350 ± 370 8,060 ± 4,990 1,500 ± 930 4,340 ± 1,540 | 3,860 ± 1,030 2,710 ± 1,520 1,370 ± 860 2,210 ± 1,250 | |
| Ovalbumin (75 µg/ml) | 5 6 7 | 1,250 ± 360 5,080 ± 1,390 3,990 ± 1,900 | 2,200 ± 690 1,880 ± 1,000 1,170 ± 400 | |
| Significant difference between ANOVA and LSD analyses: *P < 0.05 | ||||
DISCUSSION
In recent years, particularly with advances in protein separation technology, many studies have focused on immunomodulatory activities of milk or colostral protein components. The results presented in this paper confirm that dietary bovine milk whey protein has significant immuno-enhancing properties in mice when compared with SPI. It is well recognized that ruminant colostrum contains immuno-regulatory components (Watson, 1990), and work in our laboratory has also shown that ovine colostral whey can regulate antibody responses in mice when given sub-cutaneously (Watson et al. 1992). However, the same enhancing effect was not seen with mice ingesting ovine colostral whey in this study. Whether bioactive components in ovine colostral whey are more vulnerable to degradation in the gut than those in milk whey and/or other possible differences (e.g. species, composition) are involved remains to be clarified. The immunomodulatory effects of milk whey protein are unlikely to be related solely to its nutritional properties as protein increstion per se was controlled.
Dietary whey protein has been reported previously to enhance the humoral immune response to sheep red blood cells as measured by plaque?forming cells in the spleen of mice (Bounous & Kongshavn, 1982, 1985: Bounous et al. 1985, 1988a), but it did not influence the cell-mediated immune response after short-term (3 weeks) feeding (Bounous & Konashavn, 1985). Our results further confirm the enhancing effect of dietary whey protein on a humoral immune response as measured by serum anti-ovalbumin levels when compared with the effect of SPI. SPI is generally considered to be a 'complete' milk-free protein source adequate for mammals including mice. The immunoenhancing effect was observed following secondary immunization, when antibody titres reached maximum levels, and lasted for at least 4 weeks. Spleen cell proliferative responses to the B cell mitogen, lipopolysaccharides and to ovalbumin were less pronounced in the current experiments than the plaque forming cell findings of Bounous & Kongshavn (1985), although the responses were generally higher in the WPC diet group. The present results indicate that dietary whey protein enhanced the cell-mediated immune response after a 5 week feeding period. This was supported by the footpad delayed-type hypersensitivity response to sheep red blood cells (a T cell-dependent phenomenon) and to a lesser extent, by the in vitro spleen cell response to concanavalin A, which showed a significant increase for the WPC diet group after 8 weeks of feeding. These findings for cell-mediated immunity seem to be at odds with the study by Bounous & Kongshavn (1985), but the discrepancy may be due to the use of different strains of mice, lengths of dietary exposure and/or immunological methods.
Regarding mechanisms underlying the immunostimulatory effeet of dietary whey protein, a role for glutathione has been proposed by Bounous & Gold (1991) and Bounous et al. (1989, 1993). Glutathione is a tripeptide thiol which plays an important role in the stability of lysosomal and other cell membranes, and in the protection of cells from the effects of radiation and oxygen radicals (Meister & Anderson, 1983). Glutathione, therefore, is crucial to the functional state or activation of many cells including both T and B lymphocytes (Chaplin & Wedner, 1978; Noelle & Lawrence, 1981; Fischman et al. 1981; Fidelus & Tsan, 1987). Unlike other edible animal and plant proteins, whey protein has substantial amounts of glutamylcysteine groups which supply the amino acid precursors necessary for the formation of glutathione, and may therefore be responsible for the immunoenhancing effect. It would be difficult to explain why glutathione, as a non-specific metabolic compound, selectively influenced the plaque-forming cell (humoral) response but not T cell-mediated responses such as delayed-type hypersensitivity and graft v. host reactions observed by Bounous & Kongshavn (1985) in the same experiment. In this conclusion, our data offer support to the hypothesis of Bounous & Kongshavn (1985) as both humoral and cell-mediated responses were found to be influenced by dietary WPC. Alternatively, it is possible that among the numerous minor protein and peptide constituents of whey there are some that directly exert specific immunomodulatory effects on the cells of the immune system (Juto, 1985; Mincheva-Nilsson et al. 1990; Watson, 1990).
The present study has confirmed the immunoenhancing properties of milk whey proteins and identified some areas worthy of further investigation. Whether future emphasis should be placed on milk whey as a food with a unique amino acid profile or on exploitation of certain key factors in whey, or both, remains to be evaluated when highly purified whey protein fractions become available.
We thank Christine Leger for excellent technical assistance and CSIR0 Dairy Research Laboratory for WPC preparation. This research was supported by a grant from the Australian Dairy Research and Development Corporation.
REFERENCES
BARTA, O., BARTA, V.D., CRISMAN, M.V., & AKERS, R.M. 1991 Inhibition of lymphocyte blastogenesis by whey. American Journal of Veterinary Research 52 247-253.
BELOKRYLOV, G.A., POPOVA, 0.YA., MOLCHANOVA, I.V., SOROCHINSKAYA, E.I. & ANOKBINA, V.V. 1992 Peptides and their constituent amino acids influence the immune response and phagoeytosis in different ways. International Journal of Immunopharmacology 14 1285-1292.
BOUNOUS, G., BARUCHEL, S., FALUTZ, J. & GOLD, P. 1993 Whey proteins as a food supplement in HIV seropositive individuals. Clinical and Investigative Medicine 16 204-209.
BOUNOUS, G., BATIST, G. & GOLD, P. 1989 Immunoenhancing property of dietary whey protein in mice: role of'glutathione. Clinical and Investigative Medicine 12 154-161.
BOUNOUS, G. & GOLD, P. 1991 The biological activity of undenatured whey proteins: role of glutathione. Clinical and Investigative Medicine 14 296-309.
BOUNOUS, G. & KONGSHAVN, P.A.L. 1982 Influence of dietary proteins on the immune system of mice. Journal of Nutrition 112 1747-1755. BOUNOUS, G. & KONGSHAVN, P.A.L. 1985 Differential effeet of dietary protein type on the B-cell and T-cell immune responses in mice. Journal of Nutrition 115 1403-1408.
BOUNOUS, G., KONGSHAVN, P.A.L. & GOLD, P. 1988 The immunoenhancing property of dietary whey protein concentrate. Clinical and Investigative Medicine 11 271-278.
BOUNOUS, G., PAPENBURG, R., KONGSHAVN, P.A.L., GOLD, P. & FLEISZER, D. 1988b Dietary whey protein inhibits the development of dimethylhydrazine induced malignancy. Clinical and Investigative Medicine 11 213-217.
BOUNOUS, G., SHENOUDA, N., KONGSHAVN, P.A.L. & OSMOND, D.G. 1985 Mechanism of altered B-cell response induced by changes in dietary protein type in mice. Journal of Nutrition 115 1409-1417.
CHAPLIN, D.D. & WEDNER, H.J. 1978 Inhibition of lectin-induced lymphocyte activation by diamide and other sulfhydryl reagents. Cellular Immunology 36 303-311.
EIGEL, W.N., BUTLER, J.E., ERNSTROM, C.A., FARRELL, H.M., HARWALKAR, V.R., JENNESS, R. & WHITNEY, R.McL. 1984 Nomenclature of proteins of cow's milk: fifth revision. Journal of Dairy Science 67 1599-1631.
FIDELITS, R.K. & TSAN, M. F. 1987 Glutathione and lymphocyte activation: a function of ageing and autoimmune disease. Immunology 61 503-508
FISCHMAN, C.M., UDEY, M.C., KURTZ, M. & WEDNER, H.J. 1981 Inhibition of lectin?induced lymphocyte activation by 2-cyclohexene-1-one: decreased intracellular glutathione inhibits an early event in the activation sequence. Journal of lmmunology 127 2257-2262.
JUTO, P. 1985 Human milk stimulates B cell function. Archives of Disease in Childhood 60 610-613.
LIEW, F.Y. 1977 Regulation of delayed-type hypersensitivity. I. T suppressor cells for delayed-type hypersensitiviy to sheep erythrocytes in mice. European Journal of Immunology 7 714-718.
MCINTOSH, G.H. 1993 Dairy proteins: their influence on colon cancer risk. Dairy Research and Development Corporation Nutrition Workshop Biographical Data (Abstract). Melbourne, 18-19 February. 14-15.
MEISTER, A. & ANDERSON, M.E. 1983 Glutathione. Annual Review of Biochemistry 52 711-760.
MINCHEVA-NILSSON, L., HAMMARSTRÖM, M.L., JUTO, P. & HAMMARSTRÖM, S. 1990 Human milk contains proteins that stimulate and suppress T lymphocyte proliferation. Clinical and Experimental Immunology 79 463-469.
NOELLE, R.J. & LAWRENCE, D.A. 1981 Determination of glutathione in lymphocytes and possible association of redox state and proliferative capacity of lymphocytes. Biochemical Journal 198 571-579.
OGRA, S.S. & OGRA, P.L. 1978 Immunologic aspects of human colostrum and milk. II. Characteristics of' lymphocyte reactivity and distribution of E-rosette forming cells at different times after onset of lactation. Journal of Pediatrics 92 550-555.
SCHALM, O.W., JAIN, N.C. & CARROLL, E.J. (Eds) 1986 Veterinary Hematology. 4th edn. Philadelphia. PA Lea & Febiger
STOECK, M., RUEGG, C., MIESCHER, S., CARREL, S., Cox, D., VON FLIEDNFR, V. & ALKAN, S. 1989 Comparison of the immunosuppressive properties of milk growth factor and transforming growth factors ß1 and ß2. Journal of Immunology 143 3258-3265.
WATSON, D.L, 1990 Immunological activity of factors in colostrum and milk. In Joint Convention Papers. Gold Coast. QLD. 6th-l0th May. pp. 81-83 (Eds C. Halais, H. Deeth, I. Fedrick, C. Jehne, G. Leith, J. Macfarlane and P. Paroz). The QLD Branch of The Australian and New Zealand Institutes of Food Science and Technology Ltd.
WATSON, D.L.. FRANCIS, G.L. & BALLARD, F.J. 1992 Factors in ruminant colostrum that influence cell growth and murine IgE antibody responses. Journal of Dairy Research 59 369-380.