Flavonoids As Antioxidant Agents | Equine Clinical Research
Vol. 19, No. 4, pp. 481-486, 1995.
©1995 Elsevier Science. Excerpt reprinted with permission.
FLAVONOIDS AS ANTIOXIDANT AGENTS: IMPORTANCE OF THEIR INTERACTION WITH BIOMEMBRANES
ANTONELLA SAIJA, MARIO SCALESE, MARIA LANZA, DANIELA MARZULLO, FRANCESCO BONINA, and FRANCESCO CASTELLI
INTRODUCTION
Flavonoids, a group of naturally occurring benzo-g-pyrone derivatives, have been shown to possess several biological properties (including hepatoprotective, anti-thrambotic, antiinflammatory, and antiviral activities), many of which may be related, partially at least, to their antioxidant and free-radical-scavenging ability.9,10 The antiradical property of flavonoids is directed mostly toward HO; and 02 - as well as peroxyl and alkoxyl radicals.11-14 Furthermore, as these compounds present a strong affinity for iron ions (which are known to catalyze many processes leading to the appearance of free radicals), their antiperoxidative activity could also be ascribed to a concomitant capability of chelating iron.15,16
DISCUSSION
In agreement with our results, quercetin is reported to exhibit the highest antiradical property toward hydroxyl and peroxyl radicals and superoxide anions, and this predominance has been well attributed to its structural characteristics.9,11,12,14 Furthermore , we have confirmed the favorable antioxidant: activity of flavonoids carrying methoxy-phenolic structures, such as hesperetin,39 compared to poly-OH-substituted flavonoids, which were demonstrated to generate potentially toxic oxygen species at biologically relevant pH.40 In our experiments, rutin has shown an antioxidant effect comparable to that of quercetin; this also occurs if the sugar moiety known to mask the antioxidant activity of a flavonoid, probably preventing its access to the lipid membranes.38 Rutin has been demonstrated to be an effective inhibitor of iron-dependent lipid peroxidation due to the formation of inert complexes with iron.15,16,41
In conclusion, the antioxidant activity of flavonoids appears to be dictated not only by their structural features but also by their location in the membrane. This result must be taken into consideration in further developments of these protective flavonoids, which could have important applications in human diseases accompanied by free radical injury.
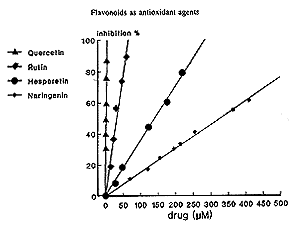 |
| Fig. 2. Inhibition of autooxidation of rat cerebral membranes by increasing concentrations of quercetin, rutin, hesperitin or naringenin. |
REFERENCES
9. Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of super oxide anions. Biochem. Pharmacol. 37:837-841; 1988.
10. Chen, Y.; Zheng, R.; Jia Z.; Ju, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 9:19-21; 1990.
11. Husain, S. R.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of Flavonoids. Phytochem. 26:2489-2491; 1987.
12. Huguet, A.L.; Manez, S.; Alacaraz, M.J. Superoxide scavenging properties of Flavonoids in non-enzymic system. Z. Naturforsch. 45c:19-24; 1990
13. Sichel. G.; Corsaro. C.; Scalia M.; DiBilio. A. J.; Bonomo, R. In vitro scavenger activity of some flavonoids and melanins against O2. Free Radic. Biol. Med. 11:1-8; 1991.
14. Torel, J.; Cillard, J.; Cillard, P. Antoxidant activity of flavonoids and reactivity with peroxy radical. Phytochem. 25:383-385; 1986.
15. Afanas'av, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V. A.; Potapovich, A. I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmocol. 38:1763-1769; 1989.
16. Morel, I.; Lescoat, G.; Cogrel, P.; Sergent. O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Phormacol. 45:13-19; 1993.
38. Ratty, A.K.; Das, N.P. Effects of flavonoids on non-enzymatic lipid peroxidation: Structure-activity relationship. Biochem. Med. Metab. Biol. 39:69-79; 1988.
39. Gyorgy, I.; Foldiak, G. Flavonoid type antioxidants: Free radical induced oxidation of silybin at neutral pH. In: Feher. J.; Blazovics, A.; Matkovics. B.; Mtzes, M.. eds. Role of free radicals in biological systems. Akademiai Kiado; 1993:37-44.
40. Hodnik, W.F.; Kung, F.S.; Roettger, W.J.; Rohmont, C.W.; Pardini, R.S. Inhibition of mitochondrial respiration andproduction of toxic oxygen radicals by flavonoids. A structure activity study. Biochem. Pharmacol. 35:2345-2354; 1986.
41. Puppo, A. Effects of flavonoids on hydroxl radical formation by Fentonn-type reactions; influence of the iron chelator. Phytochemistry. 31:85-88; 1992.
42. Parasassi, T.; Martelucci, I.; Conte, F.; Messina, B. Drug membrane interactions: Silymarin, silibyn and microsomal membranes. Cell. Biochem. Funct. 2:85-88, 1984.