Absorbic Acid and Connective Tissue Research
Subcellular Biochemistry, Volume 25: Ascorbic Acid: Biochemistry and Biomedical Cell Biology
Edited by J. Robin Harris. Plenum Press, New York, 1996.Reprinted with permission, ©1996.
Ascorbic Acid and Connective Tissue
Ivonne Pasqualli Ronchetti, D. Quaglino, Jr., and G. Bergamini
1. SCURVY AND VITAMIN C
Observations of deficient wound healing in sailors suffering from scurvy have been reported by explorers and physicians since the sixteenth century, together with the observation that citrus could have curative properties. Thereafter, Wolbach and Howe (1926) found that in scorbutic guinea pigs there was a deficient production of intercellular matrix which could be reversed by administration of citrus. The discovery, isolation, and chemical characterization of vitamin C was performed in the early thirties. Since then, several studies have been carried out with the aim of characterizing the cellular and matrix defects in scurvy and the effect of vitamin C on the healing process in species unable to synthesize ascorbic acid, such as guinea pigs and humans. Several models have been proposed, including animals made scorbutic during fetal development (Rivers et al., 1970) and postnatal growth (Barnes et al., 1970), and cultured organs and cells grown on chemically defined media in the absence (Jeffrey and Martin, 1966) or in the presence of various concentrations of ascorbic acid (Russell and Manske, 1991).The great majority of these studies, however, were performed on vitamin C-deficient guinea pigs and pointed to the delay and to the histological, biochemical, and mechanical features of healing of skin wounds or after laparatomy. It was observed that vitamin C-deficient animals exhibited persistent hemorrhaging; impaired production of granulation tissue, such as reduced vessel formation and collagen production; and slowed gain in wound strength (Lanman and Ingalls, 1937; Hunt, 1940; Hartlett et al. 1942: Bourne, 1944). These early data were confirmed in a recent study on pregnant sows with a hereditary defect in synthesizing ascorbic acid, in which severe pathological alterations were observed in the uterus and in the placenta as well as in the fetuses on administration of a diet depleted of vitamin C. These changes consisted of hemorrhages, hematomas, and general edema in both placenta and fetuses and in impaired ossification of the fetus skeleton (Wegger and Palludan, 1994).
The distribution of ascorbic acid in wounded and intact skin of guinea pigs was investigated in an attempt to elucidate requirements of vitamin C during tissue repair, and the authors reached the conclusion that in the early stage of tissue regeneration there is a gradient distribution of ascorbic acid in areas surrounding the wound depending on the local physiological requirement (Kim et al., 1994).
Experimental vitamin C deficiency in humans was assayed in the early forties by Crandon (1940), who underwent a skin incision after six months on a diet essentially free of ascorbate. Failure in the reparative process, together with deficient formation of inter-cellular matrix and vascular elements, were histologically observed in a biopsy of the wound taken ten days after a skin incision. Similar results were later confirmed by Wolfer and coworkers (1947).
Since then, numerous investigations have demonstrated that in vitamin C deficiency the principal failure of wound healing was impaired synthesis and secretion of collagen (Robertson and Schwartz, 1953).
2. COLLAGEN AND VITAMIN C
The great majority of studies on the effects of vitamin C have been performed in vitro; on cultured cells of different origin and have pointed to the role of ascorbic acid in collagen synthesis, maturation, and secretion.In the early sixties, with the development of electron microscopy, Ross and Benditt (1962, 1965) observed in experimental scurvy a defective progression of labeled proline through the altered cysternae of the rough endoplasmic reticulum to the Golgi and the matrix. These results might be interpreted as impaired processing of collagen and storage of underhydroxylated molecules within the endoplasmic reticulum of fibroblasts. which were stimulated to synthesize collagen during wound healing in the absence of vitamin C. An enormous number of studies performed in the sixties demonstrated that the principal failure in wound healing during vitamin C deficiency is the scarce synthesis and secretion of collagen due to impaired hydroxylation of proline residues in collagen types I and III (Gould and Woessner, 1957; Gould, 1958).
Synthesis and maturation of interstitial collagens up to their final cross-linking into insoluble cross-banded fibrils is not the subject of this presentation, but it is necessary to mention some of the steps involved in the maturation of collagen molecules in order to understand the effects of vitamin C. Like the great majority of secreted proteins, procollagen molecules are synthesized in the rough endoplasmic reticulum and require posttranslational modifications before being extruded from the cell. However, collagen is a rather peculiar protein. First of all, the molecule is formed by three polypeptide chains that assume a specific helical conformation due to the high content of glycine which occupies every third position along most of the length of the three polypeptide chains. Moreover, collagen is characterized by the presence of hydroxyproline and hydroxylysine formed by specific hydrolases during the molecule assembly; furthermore, some of the hydroxylysine residues undergo O-galactosyl and O-galactosyl-b-glycosyl substitution. All these post-translational modifications are necessary in order for collagen to be secreted from the cells as procollagen.
In the extracellular space, procollagen is further modified by enzymes which cut the C- and N-terminal portions of the molecule and make tropocollagen suitable for self-assembly into banded fibrils. The last enzymatic modification of the collagen molecule is by lysyl oxidase, which initiates a series of reactions leading to the formation of stable intermolecular cross-links. As far as lysyl oxidase is concerned, in vivo, this enzyme activity in rat skin does not seem to be significantly affected by excess of vitamin C in the diet; on the contrary, lysyl oxidase was inhibited in a concentration-dependent manner in an in vitro assay in which lysyl oxidase activity of chick embryo bones was measured in the presence of increasing concentrations of ascorbic acid (Quaglino et al., 1991). A similar reduction was also observed by Faris et al. (1984) in cultures of rabbit aortic smooth muscle cells.
With time, collagen undergoes other modifications such as glycosylation and additional intermolecular cross-linking, that do not suggest a role for vitamin C, but may be particularly relevant in pathological processes.
2.1. Collagen Hydroxylation
As already mentioned, collagen contains the unique amino acids hydroxyproline and hydroxylysine, which are necessary for the stability of the molecule and for its complete maturation. The synthesis of these amino acids occurs posttranslationally during the assembly of the polypeptidic chain (Uitto and Prockop, 1974), is independent of the age (Brinckmann et al., 1994), and is catalyzed by prolyl and lysyl hydroxylases in the presence of oxygen, a-ketoglutarate, ferrous ions, and ascorbic acid (Hutton et al., 1967; Kivirikko and Prockop, 1967). Ascorbic acid has been found to be specifically required for the decarboxylation of a-ketoglutarate in the prolyl-4-hydroxylase reaction, where it may act as a com pound necessary for the reduction of enzyme-bound ferric iron formed during proline hydroxylation (Yu et al., 1988). In fact, ascorbate is not stoichiometrically consumed during prolyl hydroxyletion (Tuderman et al., 1977), and the reaction may continue for several cycles in the absence of ascorbate, but then the reaction ceases and vitamin C is required as a quite specific compound to reactivate the enzyme (Myllyla et al., 1978). Hydroxylation of a number of proline and lysine residues, at specific sites of the nascent collagen molecule (Uitto and Prockop, 1974), is necessary for the polypeptidic chain to undergo peculiar conformation and glycosylation of some of the hydroxylated lysyl residues; all these steps are necessary for the secretion of the procollagen molecule.Hydroxylation of prolyl and lysyl residues may also occur in vitro. Protocollagen, the unhydroxylaled form of collagen, was isolated from cells cultured in the presence of the iron chelator a,a-dipyridyl; moreover, the in vitro hydroxylation of prolyl residues by prolyl hydroxylase was shown to be dependent on the structure of prolyl-containing substrate (Berg and Prockop, 1973a). Therefore, during the formation of procollagen, prolyl and lysyl hydroxylases serve to prepare the molecule to assume the correct conformation necessary for its thermal stability and secretion (Uitto and Prockop, 1974). In fact, underhydroxylated and underglycosylated collagen has been shown to be retained within the cell and to accumulate into large cyloplasmic vacuoles (Koss and Benditt, 1965; Olsen and Prockop, 1974: Harwood et al., 1975). The persistence within the endoplasmic reticulum could be explained, at least partially, by the fact that the underhydroxylated chains undergo a delay in the triple helix formation and may stably associate with protein disulfide isomerase, a multifactoral endoplasmic reticulum resident enzyme, which is the b-subunit of prolyl-4-hydroxylase (Bassuk and Berg, 1989). Therefore, prolyl-4-hydroxylase specifically binds the non-triple helical procollagen chain, playing a role in its intracellular retention (Olsen et al., 1973). Similar retention has been observed in a strain of fibroblasts harboring a deletion of 180 amino acids in the pro-a2(I) chain, which causes a delay in the molecule folding into the triple helix and in collagen maturation (Chessler and Byers, 1992).
In the absence of vitamin C, underhydroxylated procollagen molecules are not only retained within cells (Dehm and Prockop, 1971), but are less stable and more temperature-sensitive(Berg and Prockop, 1973b). Procollagens with different hydroxyproline content were shown to be sensitive to pepsin digestion at temperatures lower than physiological, and the phenomenon was directly related to the extent of hydroxylation. Therefore, at 37EC, hydroxyproline-deficient molecules might not be in triple helical conformation within cells and could be most sensitive to proteases (Rosenbloom et al., 1973). This may imply that, in vitamin C deficiency, the impaired collagen production is partly due to its cellular retention and partly to its denaturation and destruction by unspecific proteases within the cell.
Cell strains isolated from patients suffering osteogenesis imperfecta, a connective tissue disorder caused by mutations in the genes encoding type I collagen and affecting procollagen chain association, were shown to increase procollagen synthesis upon addition of ascorbic acid to the culture medium, as well as the synthesis of BiP, an hsp70-related stress protein, which was found to bind pro-a1(I) chains stably (Chessler and Byers, 1993). This could be a mechanism for retaining genetically altered and abnormally hydroxylated (and/or glycosylated) collagen molecules inside cells. As BiP synthesis is stimulated by ascorbic acid, this could be an additional mechanism for preventing secretion of abnormally configured collagen molecules.
2.2. Collagen Gene Expression
Human dermal fibroblasts and rabbit articular chondrocytes were shown to produce higher amounts of collagen in the presence of ascorbic acid in vitro (Hata and Senoo, 1989; Hering et al., 1994); moreover, ascorbic acid was shown to overcome the reduced proliferative capacity and to ameliorate the reduced collagen synthesis of elderly human fibroblasts (Phillips et al., 1994).A stimulatory effect of ascorbate has been largely demonstrated on the synthesis of collagen types I and III and recently shown for collagen types II and X in chondrocytes (Leboy et al., 1989; Hering et al., 1994) and for collagen type IV in cultured rat skin epidermal cells (Ohkura et al., 1990). On the contrary, a negative effect of vitamin C has been described for collagen types V and VI in cultured bovine aortic smooth muscle cells (Leushner and Haust, 1986).
These data might be explained by the fact that ascorbic acid seems to play a role in collagen synthesis also at the level of gene expression and/or mRNA stability in cultured fibroblasts and chondrocytes (Lyons and Schwartz, 1984; Geesin et al., 1988; Sandell and Daniel, 1988; Quaglino et al., 1989; Kurata and Hata, 1991; Kurata et al., 1993; Phillips et al., 1994), as well as in vivo (Quaglino et al., 1991). However, the mechanisms involved are still not completely known. It is worthwhile mentioning that the majority of studies of the effect of vitamin C on collagen gene expression have been made in the presence of connective tissue-modulating growth factors, such as epidermal growth factor (EGF), transforming growth factor (TGF-b), and fibroblast growth factor (FGF) (Hata et al., 1988; Appling et al., 1989; Kurata and Hata, 1991; Phillips et al., 1992; Geesin et al., 1993).
During the first weeks of vitamin C deprivation, scorbutic animals exhibit reduced food intake, which correlates with the rate of weight loss and with the decrease of collagen and proteoglycan synthesis (Chojkier et al., 1983). Later it was shown, however, that reduced collagen mRNA expression and synthesis can be observed in several tissues of vitamin C-deficient guinea pigs, which may not be simply related to the reduced food intake or to the role of vitamin C in the hydroxylation of proline residues in collagen; therefore, other more complex mechanisms and interactions among different cell products seem to be involved (Gosiewska et al., 1994). In vitamin C-deficient animals, elevated levels of IGF (insulin growth factor) mRNA and of IGFBP (insulin growth factor binding protein) mRNA have been identified, which seem to be responsible for the inhibition of the IGF-I-dependent functions. Removal of these inhibitors by specific antibodies restores collagen gene expression. Therefore, vitamin C deficiency seems to induce the synthesis of inhibitors of IGF dependent functions, such as collagen gene expression (Goldstein et al., 1989, Peterkofsky et al.,. 1994; Gosiewska et al., 1994).
Rather interestingly, vitamin C has been shown to stimulate transcription of the gene and accumulation of mRNA for the pro-a1(I) chain but has failed to stimulate transcription and increase of the mRNA for the pro-a2(I) chain in fibroblasts from a patient with a2(I)-chain defective Elhers Danlos syndrome (Hata and Senoo, 1992; Kurata et al., 1993). This may indicate the presence of different regulatory elements responsible for transcriptional activation by vitamin C in pro-a1 (I) and pro-a2 (I) genes in normal fibroblasts (see also Chapter 3).
In a study we performed on the effects of excess of vitamin C on collagen of growing rats, an increase or collagen deposition as well as mRNA expression was observed in the aorta after more than 10 days of treatment (Quaglino et al., 1991).
According to some authors, ascorbic acid might stimulate collagen gene expression through lipid peroxidation; vitamin C, in fact, may induce lipid peroxidation with the formation of aldehydic compounds, and some of these, such as malondialdehyde have been shown to stimlulate collagen production and raise procollagen a1 (I) mRNA levels in vitro (Brenner and Chojkier, 1987; Chojkier et al., 1989). Moreover, both lipid peroxidation and collagen production induced by ascorbic acid have been shown to be inhibited by a-tocopherol, a lipophilic antioxidant, or by iron chelators, suggesting that the two processes are correlated and that an appropriate redox state might be an important mechanism in controlling collagen gene expression in vivo (Geesin et al., 1991).
More recent data, however, seem to point to a different interpretation of the role of the ascorbate-induced lipid peroxidation on collagen gene expression (Darr et al., 1993). It has been suggested that lipid peroxidation and collagen synthesis are coincidental but dissociated, as metal chelators used to abolish the iron-ascorbic acid-induced lipid peroxidation may also inhibit prolyl hydroxylase and, as a consequence, collagen production. Moreover, cell-impermeable iron chelators have been found to be good inhibitors of ascorbate-mediated lipid peroxidation but ineffective in inhibiting collagen synthesis (Darr et al., 1993).
In any case, almost all studies on the promoting effect of lipid peroxidation by vitamin C were performed in vitro; the effect of vitamin C-induced lipid peroxidation might not be so relevant in vivo. Under physiological conditions, most of the iron is bound to proteins, and vitamin C might prevent lipid peroxidation instead of stimulating it (Mukhopadhyay et al., 1997). Moreover, the role of ascorbic acid in vivo is generally considered to be one of cellular defense against oxygen toxicity and lipid peroxidation (Chakraborty et al., 1994) through a mechanism of free radical scavenging followed by its conversion to dehydroascorbic acid. Collagen indeed is susceptible to fragmentation by superoxide anion with liberation of small hydroxyproline-containing peptides (Monboisse and Borel, 1992), and, in vivo, vitamin C could protect collagen from degradation by hydroxyl radicals in the presence of oxygen.
Therefore, collagen synthesis, maturation, and secretion as well as collagen degradation are tightly bound processes, and vitamin C seems to be involved at different levels of the whole process.
3. ELASTIN AND VITAMIN C
From the early studies on the effect of ascorbic acid on collagen production, it was observed that vitamin C deficiency did not affect elastin synthesis and secretion, although it greatly influenced the degree of its proline hydroxylation. In the presence of vitamin C, hydroxyproline in elastin accounts for about one third of the total amino acid content (Uitto et al., 1976), and proline/hydroxyproline ratio in elastin is approximately 1:1; on the contrary, in both skin and aortas of vitamin C-deprived guinea pigs, the proline/hydroxyproline ratio in elastin was 20:1 (Barnes et al., 1970). Though underhydroxylated collagen cannot be excreted from cells, underhydroxylated elastin is secreted at a normal rate (Rosenbloom and Cywinski, 1976) and, similar to collagen, in a colchicine-sensitive way (Uitto et al., 1976).
An influence of vitamin C on elastin synthesis was described by Scott-Burden and coworkers (1979), who found that heart smooth muscle cells in vitro incorporated radioactive valine, an amino acid prevalent in elastin, to a greater quantity in the absence of than in the presence of ascorbic acid. Moreover, the elastin produced and secreted in the absence of vitamin C underwent the cross-linking process in the extracellular space more rapidly than that produced in the presence of ascorbic acid (Scott-Burden et al., 1979). Similar findings were published by DeClerck and Jones (1980), who found that the amount of insoluble elastin in the extracellular space was inversely proportional to the ascorbic acid concentration in the medium. Therefore, proline hydroxylation in elastin is not necessary for secretion or for molecule assembly and cross-linking; on the contrary, underhydroxylation in vitamin C deficiency seems to favor elastin assembly and its stabilization into the polymer. Moreover, hyperhydroxylated elastin, produced in vitro in the presence of ascorbic acid, was shown to contain more free lysine residues and to turn over more rapidly (Dunn and Franzblau, 1982). At physiological temperatures, both in vivo and in vitro, tropoelastin molecules undergo a process of self-assembly into fibrillar structures called coacervates (Cox et al., 1974; Volpin and Pasquali-Ronchetti, 1977; Bressan et al., 1983, 1986). This phenomenon also seems to happen in vivo and to be a prerequisite for enzymatic cross-linking of tropoelastin molecules, through the oxidative deamination of e-amino groups of lysine residues on adjacent molecules by the enzyme lysyl oxidase (Narayanan et al., 1978). The inhibition of the molecular assembly by hyperhydroxylation of proline would lead to a less cross-linked stable polymer (Urry et al., 1979).
In order to investigate whether an excess of ascorbic acid could modify in vivo the assembly of the elastic fibers, we treated growing chicks and rats with excess of vitamin C in their diet and drinking water, respectively (Quaglino et al., 1991). Animals were killed after various treatment times and the aorta examined by electron microscopy, in situ hybridization, and biochemical methods. After 30 days treatment, the ultrastructural organization of the aortic elastic fibers appeared to be significantly affected by vitamin C treatment compared to control animals (Fig. 1); in situ hybridization revealed a decreased expression of elastin mRNA on slices from the aorta of vitamin C-treated rats (Fig. 2); moreover, stereological measurements on electron micrographs showed a significant increase in collagen and decrease in the elastin content in the aortic wall of treated animals (Fig. 3)
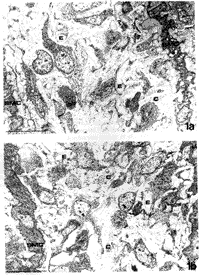 |
| FIGURE 1.Electron microscopy of 30-day chick aorta. Animals were fed a standard diet (a) or a diet supplemented with 0.2% ascorbic acid from hatching (b). Chick aorta is composed of layers of smooth muscle cells (SMC) among which there are several elastic fibers (E) and a few collagen bundles (C). Vitamin C seems to cause an increase in collagen bundle deposition and a decrease in elastic fiber assembly. Bar: 1 Fm. |
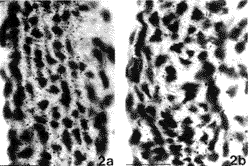 |
| FIGURE 2. In situ hybridization of rat aorta in 30-day old animals grown, at weaning, on a standard diet (a) or on a standard diet plus 10% ascorbic acid added to the water (b). Sections have been hybridized with a 1.0 kb cDNA fragment (cHE-4) corresponding to exons of 18 to 36 of human elastin and exposed for autoradiography. Animals treated with an excess of vitamin C show a decreased expression of elastin mRNA compared to control animals. Bar: 10 Fm. |
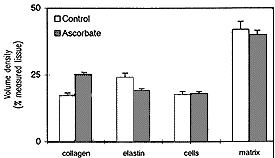 |
| FIGURE 3. Morphometric analysis of the rat aorta in 50-day old animals grown, at weaning, on a standard diet plus 10% ascorbic acid added to the water. Treatment causes an increased deposition of collagen bundles and reduced amounts of elastic fibers, whereas no significant changes were observed in the cellular component or in the remaining extracellular matrix. |
4. MATRIX GLYCOPROTEINS AND VITAMIN C
Relatively few studies are available on the effects of vitamin C on synthesis and secrction of matrix molecules apart from collagen and elastin; however, in a number of studies occasional mention is found of fibronectin, proteoglycans, bone matrix glycoproteins, and elastin-associated fibrillin (Schwartz et al., 1982; Kielty and Shuttleworth, 1993).Ascorbic acid has been shown to stimulate in vitro differentiation and production of matrix molecules by adipocytcs (Taylor and Jones, 1979), fat-storing cells (Senoo and Hata, 1994), chondrocytes (Leboy et al., 1989; Aulthouse, 1994), myoblasts (Nandan et al., 1990; Mitsumoto et al., 1994), and osteoblasts (Franceschi and Iyer, 1992). In this latter case, vitamin C has been shown to be taken up by the cell through a specific transport system (Wilson and Dixon, 1989) and to influence osteoblast differentiation in a rather unusual way. The expression of the osteoblast phenotype is regulated by a series of factors, including growth factors, glucocorticoids, parathyroid hormone, and 1,25-dihydroxyvitamin D3; however, differentiation and mineralization seem to require the presence of an extracellular collagen matrix. Vitamin C has been shown to be necessary both for the production of the collagen matrix and for the expression of osteoblast markers, such as alkaline phosphatase and osteocalcin, whereas it seems to have no effect on the level of osteopontin mRNA (Franceschi and Iyer, 1992). In recent years, a number of papers have pointed to the effect of ascorbic acid on the differentiation of bone cells. Ascorbic acid has also been shown to enhance the effect of retinoic acid on mRNA expression of pro-a1(I) collagen and of alkaline phosphatase in an immortalized strain of rat osteoblasts in culture (Choong et al., 1993). Ascorbic acid was found to stimulate cell proliferation, together with collagen, non-collagenous protein, and alkaline phosphatase synthesis, in pig bone cells in culture when added after cell confluence, suggesting that it may interfere with cell differentiation (Denis et al., 1994).
Ascorbic acid, in association with b-glycerophosphate, was found to stimulate matrix mineralization by inducing an increase of neutral metalloproteinase in matrix vesicles, which may be able to degrade proteoglycans favoring mineral precipitation (Brooks et al., 1994). Once again, vitamin C, b-glycerophosphate, and dexamethasone induced an increases of the mRNA level for collagen type 1, osteocalcin, bone sialoprotein, and alkaline phosphatase in association with the development of bone nodules in an in vitro system (Malaval et al., 1994). In all these studies, ascorbic acid seemed to act as a promoter for collagen synthesis and secretion, whereas subsequent cell-matrix interactions seemed to influence cell shape, metabolism, and differentiation (Aulthouse, 1994). In fact, bone protein synthesis was blocked by inhibitors of collagen triple helix formation (Franceschi and Iyer, 1992), but these inhibitors were ineffective if added after a certain amount of normally hydroxylated collagen had been produced (Franceschi et al., 1994). This could mean that bone cell differentiation depends on vitamin C for the synthesis and secretion of the first collagen matrix and that it may continue also in the absence of ascorbic acid due to the already established cell-matrix interactions.
Addition of ascorbate to cultured calf aortic smooth muscle cells was shown to increase collagen secretion together with fibronectin and proteoglycans and the phenomenon was associated both with changes in cell morphology, from elongated to polygonal, and with an increase of the cell growth rate (Schwartz et al., 1982). These findings could be the result of the first deposition of a matrix collagen, stimulated by ascorbic acid, followed by changes in cell metabolism that are regulated by cell-collagen interactions.
The effect of ascorbic acid on the production of proteoglycans is rather controversial. Edward and Oliver (1983) found that both hyaluronate and sulfated glycosaminoglycan synthesis by human skin fibroblasts was affected by vitamin C. Kao and coworkers (1990) found that ascorbic acid stimulates the production of glycosaminoglycans in cultured fibroblasts, whereas Pacifici (1990) observed that in chick chondrocyte cultures the secretion of keratin sulfate and chondroitin sulfate-containing proteoglycans was not affected by ascorbic acid in the culture medium.
In a recent study, vitamin C was shown to negatively influence the synthesis of aggrecan and to abolish the lag phase for decorin synthesis in cultured rabbit articular chondrocytes (Hering et al., 1994). The production of laminin and fibronectin was also shown to be increased by vitamin C added to the cultured bovine trabecular meshwork cells (Yue et al., 1990), suggesting a possible influence on cellular adhesion molecules.
5. REFERENCES
Appling, W.D. O`Brien, W.R., Johnston, D.A., and Duvic, M., 1989, Synergistic enhancement of type I and III collagen production in cultured fibroblasts by transforming growth factor-b and ascorbate. FEBS Lett. 250:541-544.Aulthouse, A.L, 1994, Prolonged exposure of human chondrocytes to ascorbic acid modifies cellular behavior in an agarose gel, Anat. ResA. 238:31-37.
Barnes, M.J., Constable, B.J., Morton, L.F., and Kodicek, E., 1970, Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Evidence for the formulation and degradation of a partially hydroxylated collagen. Biochem. J. 119:575-585.
Bartlett, M.K., Jones, C.M., and Ryan, A.E., 1942, Vitamin C in wound healing. I. Experimental wounds in guinea pigs, N. Eng. J. Med. 226:469-473
Bassul, J.A., and Berg, R.A., 1989, Protein disulfide isomerase, a multifunctional endoplasmic reticulum protein, Matrix 9:244-258.
Berg, R.A., and Prockop, D.J., 1973a, Purification of 14C-protocollagen and its hydroxylation by prolyl-hydroxylase, Biochemistry 12:3395-3401.
Berg, R.A., and Prockop, D.J., 1973b, The thermal transition of a non-hydroxylated form of collagen. Evidence for the role of
hydroxylproline in stabilizing the triple helix of collagen, Biochem. Biophys. Res. Comm. 52:115-120.
Bourne, G.H., 1944, Effect of Vitamin C deficiency on experimental wounds. Tensile strength and histology, Lancet 1:688-692.
Brenner, D.A., and Chojkier, M., 1987, Acetyldahyde increases collagen gene transcription in cultured fibroblasts, J. Biol. Chem. 262:17690-17696.
Bressan, G.M., Castellani, I., Giro, G.M., Volpin, D., Fornieri, C., and Pasquali-Ronchetti, I., 1983, Banded fibers in tropoelastin coacervates at physiological temperatures, J. Ultrastruct. Res. 82:335-340.
Bressan, M.G., Pasquali-Ronchetti, I., Fornieri, C., Mattioli, F., Casstelani, I., and Volpin, D., 1986, Relevence of aggregation properties of tropoelastin to the assembly and structure of elastin fibers, J. Ultrastruct, Mol, Struct. Res. 94:209-216.
Brinckmann, J., Bodo, M., Brey, M., Wolff, H.H., and Muller, P.K., 1994, Analysis of the age related composition of human skin collagen and collagens synthesizid by fibroblast cultures, Arch. Dermatol. Res. 286: 391-395.
Brooks, B.P., Qiao, M., Howell, D.S., and Boyan, B.D., 1994, Matrix vesicles produced by osteoblast-like cells become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic acid, Calcif. Tissue. Int. 54:399-408.
Chakraborty, S., Nandy, A., Mukhopadhyay, M., and Mukhopadhyay, C.K., Free Rad. Biol. Med. 16:417-426.
Chessler, S.D., and Byers, P.H., 1992, Defective folding and stable association with protein disulfide isomerase/prolyl hydroxylase of type I: the Gly-X-Y repeat pattern, J. Biol. Chem. 267:7751-7757.
Chessler, S.D., and Byers, P.H., 1993, BiP binds type I procollagen pro-alpha chains with mutations in the carboxyl-terminal propeptide synthesized by patients with osteogenisis imperfecta, J. Biol. Chem. 268:18226-18233.
Chojkier, M., Spanheimer., R., and Peterkofski, B., 1983, Specifically decreased procollagen biosynthesis in scurvy dissociated from an effect on proline hydroxylation and correlated with body weight loss, J. Clin. Invest. 72:826-835.
Chojkier, M., Houglum, K., Solis-Herruzo, J., and Brennar, J.A., 1989, Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts, J. Biol. Chem. 264:16957-16962.
Choong, P.F.M., Martin, T.J., and Ng, K. W., 1993, Effects of ascorbic acid, calcitrol, and retinoic acid on the differentiation of preosteoblasts, J. Orthop. Res. 11:638-647.
Cox, B.A., Starcher, B.C., and Urry, D.W., 1974, Coacervation of tropoelastin results in fiber formation, J. Biol. Chem. 249:997-998.
Crandon, J.H., Lund, C.C., and Dill, D.B., 1940, Experimental human scurvy, N. Eng. J. Med. 223:353-369.
Darr, D., Combs, S., and Pinnell, S., 1993, Ascorbic acid collagen synthesis: Rethinking a role for lipid peroxidation, Arch. Biochem. Biophys. Acta. 307:331-335.
Declerk, Y.A., and Jones, P.A., 1980, The effect of ascorbic acid on the nature and production of collagen and elastin by rat smooth muscle cells, Biochem. J. 186:217-2125.
Dehm, P., and Prockop, D.J., 1971, Synthesis and extrusion of collagen by freshly isolated cells from chick embryo tendon, Biochim. Biophys. Acta. 240:358-369.
Denis, I., Pointillart, A., and Lieberherr, M., 1994, Cell stage dependent effects of ascorbic acid on cultured porcine bone cells, Bone Miner. 25:149-161.
Dunn, D.M., and Franzblau, C., 1982, Effects of ascorbate on insoluble elastin accumulation and crosslink formation in rabbit pulmonary artery smooth muscle cultures, Biochemistry 18:4195-4202.
Edward, M., and Oliver, R.F., 1983, Changes in the synthesis,distribution and sulphation of glycosaminoglycans of cultured human skin fibroblasts upon ascorbate feeding, J. Cell Sci. 64:245-254.
Faris, B., Ferrera, R., Toselli, P., Nambu, J., Cionnerman, W.A., and Franzblau, C., 1984, Effect of varying amounts of ascorbate on collagen, elastin and lysyl oxidase synthesis in aortic smooth muscle cell cultures, Biochim. Biophys. Acta. 797:71-75.
Franceschi, R.T,. and Iyer, B.S., 1992, Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-EI cells, J. Bone Miner. Res. 7:235-246.
Franceschi, R.T, Iyer, B.S., and Cui, Y., 1994, Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res. 9:843-854.
Geesin, J.C., Darr, D., Kaufmen, R., Murad, S., and Pinnell, S.R., 1988, Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblasts, J. Invest. Dermatol. 90:420-424.
Geesin, J.C., Hendricks, L.J., Falkenstein, P.A., Gordon, J.S., and Berg, R.A., 1991, Regulation of collagen synthesis by ascorbic acid: Characterization of the role of ascorbate-stimulated lipid peroxidation, Arch. Biochem. Biophys. 290:127-132.
Geesin, J.C., Brown, L.J., Gordon, J.S., and Berg, R.A., 1993, Regulation of collagen synthesis in human dermal fibroblasts in contracted collagen gels by ascorbic acid, growth factors and inhibitors of lipid peroxidation, Exp. Cell Res. 206:283-290.
Goldstein, R.H., Poliks, C.F., Pilch, P.F., Smith, B.D., and Fine, A., 1989, Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts, Endocrinology 124:964-970.
Cosiewska, A., Wilson, S., Kwon, D., and Peterkowsky, B., 1994, Evidence for an in vivo role of insulin-like growth factor-binding protein-1 and -2 as inhibitors of collagen gene expression in vitamin C-deficient and fasted guinea pigs, Endocrinology 134:1329-1339.
Gould, B.S., 1958, Biosynthesis of collagen. III. The direct action of ascorbic acid on hydroxyproline and collagen formation in subcutaneous polyvinyl sponge implants in guinea pigs, J. Biol. Chem. 232:637-649.
Gould, B.S., and Woessner, J.F., 1957, Biosynthesis of collagen. The influence of ascorbic acid on the proline, hydroxyproline, glycine and collagen content of regenerating guinea pig skin, J. Biol. Chem. 266:289-300.
Harwood, R., Grant, M.E., and Jackson, D.S., 1975, Studies on the glycosylation of hydroxylysine residues during collagen biosynthesis and the subcellular localization of collagen galactosyltransferase and collagen glucosyltransferase in tendon and cartilage cells, Biochem. J. 152:291-302.
Hata, R.I., and Senoo. H., 1989, L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissue-like substance by skin fibroblasts, J. Cell. Physiol. 138:8-16.
Hata, R.I., and Senoo, H., 1992, Extracellular matrix system regulates cell growth, tissue formation, and cellular functions, Tissue Cult. Res. Commun. 11:337-343.
Hata, R.I., Sunada, H., Arai. K., Sate, T., Ninomiya. T., Nagai, Y., and Senoo, H., 1988, Regulation of collagen metabolism and cell growth by epidermal growth factor and ascorbate in cultured human skin fibroblasts, Eur. J. Biochem. 173:261-267.
Hering, T.M., Kollar, J., Huynh, T.D., Varelas, J.B., and Sandell, L.J., 1994, Modulation of extracellular matrix gene expression in bovine high-density chondrocyte cultures by ascorbic acid and enzymatic resuspension, Arch. Biochem. Biophys. 314:90-98.
Hunt, H.A., 1940, The role of vitamin C in wound healing, Br. J. Surg. 28:436-461.
Hutton, J.J., Tappel, A.L., and Udenfriend, S., 1967, Cofactor and substrate requirements of collagen proline hydroxylase, Arch. Biochim. Biophys. 118:231-240.
Jeffry, J.J., and Martin, G.R., 1966, The role of ascorbic acid in the biosynthesis of collagen. I. Ascorbic acid requirements by embryonic chick tibia in tissue culture, Biochim. Biophys. Acta. 121:269-280.
Kao, J., Huey, G., Kao, R., and Stern, R., 1990, Ascorbic acid stimulates production of glycosaminoglycans in cultured fibroblasts, Exp. Mol. Pathol. 53:1-10.
Kielty, C.M., and Shuttleworth, C.A., 1993, Synthesis and assembly of fibrillin by fibroblasts and smooth muscle cells, J. Cell. Sci. 106:167-173.
Kim, M., Otsuka, M., Yu, K., Kurara. T., and Arakawa, N., 1994, The distribution of ascorbic acid and in wounded dorsal skin of guinea pigs, Int. J. Vitam. Nutr. Res. 64:56-59.
Kivirikko, K.I., and Prockop, J.J., l967, Enzymatic hydroxylation of proline and lysine in procollagen, Proc. Nat. Acad. Sci. USA 57:782-789.
Kurata, S., and Hata. R., 1991, Epidermal growth factor inhibits transcription of type I collagen genes in cultured human skin fibroblasts in the presence and absence of L-ascorbic acid 2-phosphate, a long lasting vitamin C derivative, J. Biol. Chem. 266:9997-10003.
Kurata, S.I.. Senoo, H., and Hata, I.I., 1993, Transcriptional activation of type I collagen genes by ascorbic acid 2-phosphate in human skin fibroblasts and its failure in cells from a patient with a2 (I)- chain defective Ehlers-Danlos syndrom, Exp. Cell Res. 206:63-71.
Lanman, T.H., and Ingalls, T.H., 1937, Vitamin C and wound healing. Experimental and clinical study, Ann. Surg. 105:616-625.
Leboy, P.S., Vaias, L., I Uschmann, B., Golub, E., Adams, S.L., and Pacifici, M., 1989, Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes, J. Biol. Chem. 264:17281-17286.
Leushner, J.R., and Haust, M.D., 1986, The effect of ascorbate on the synthesis of minor (non-interstital) muscle cells, Biochim. Biophys. Acta. 883:284-292.
Lyons, B.L., and Schwartz, R.I., 1984, Ascorhate stimulation of PAT cells causes an increase in transcription rates and a degradation in rates of procollagen mRNA, Nucl. Acids Res. 12:2569-2579.
Malaval, I., Modrowski, D., Gupta, A.K., and Aubin, J.E., 1994, Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures, J. Cell Physiol. 158:555-572.
Mitsomito, Y., Liu, Z., and Klip, A., 1994, Long-lasting vitamin C derivative, ascorbic acid 2-phosphate increases myogenin gene expression and promotes differentiation in L6 muscle cells, Biochim. Biophys. Res. Commun. 199:394-402.
Monboisse, J.C., and Borrel, J.P., 1992, Oxidative damage to collagen, in Free Radicals and Aging (J. Emerit and B. Chance, eds.) pp.323-327. Berkhauser Verlag, Basel.
Mukhopadhyay, M., Mukhopadhyay, C.K., and Chatterjee, I.B., 1993, Protective effect of ascorbic acid against lipid peroxidation and oxidative damage in cardiac microsomes, Mol. Cell. BiochemA 126:69-75.
Myllila, R., Kuutti-Savolilanin, E.R., and Kivirikko, K.I., 1978, The role of ascorbate in the prolyl-hydroxylase reaction, Biochem. Biophys. Res. Commun. 83:441-448.
Nandan, D., Clarke, E.P., Ball, E.H., and Sanwall, B.D., 1990, Ethyl-3,4-dihydrobenzoate inhibits myoblast differentiation: Evidence for an essential role of collagen, J. Cell. Biol. 110:1673-1679.
Narayanan, A.S., Page, K.C., Kuzan. F., and Cooper, C.G., 1978, Elastin cross-linking in vitro. Studies on factors influencing the formation of desmosines by lysyl oxidase action on tropoelastin, Biochem. J. 173:857-862.
Ohkura, K., Fujii, T., Konishi, R., and Terada, H., 1990, Increased attachment and confluence of skin epidermal cells in culture induced by ascorbic acid. Detection by permeation of trypan blue across cultured cell layers. Cell. Struct. Funct. 15:143-150.
Olsen, B.R., and Prockop, D.J., 1974, Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc. Natl. Acad. Sci. USA 71:2033-2037.
Olsen, B.R., Berg, R.A., Kishida. Y., and Prockop, D.J., 1973, Collagen synthesis: Localization of prolyl hydroxylase in tendon cells detected with ferritin-labeled antibodies, Science 182:825-827.
Pacifici, M., 1990, Independent secretion of proteoylycans and collagens in chick chondrocyte cultures during acute ascorbic acid treatment. Biochem. J. 272:193-199.
Peterkofsky, B., Gosiewska, A., Kipp, D.E., Shah, V., and Wilson, S., 1994, Circulating insulin-like growth factor binding proteins (IGFBPs) 1 and 2 induced in vitamin C-deficient or fasted guinea pigs inhibit IGF-I action in cultured cells, Growth Factors 10:229-241.
Phillips, C.L.,Tajima, S., and Pinnell, S.R., 1992, Ascorbic acid and transforming growth factor-b1 increase collagen biosynthesis via different mechanisms: Coordinate regulation of pro a(I) and pro a1(III) collagens. Arch. Biochem. Biophys. 295:397-403.
Phillips, C.L., Combs, S.B., and Pinnell, S.R., 1994, Effects of ascorbic acid on proliferation and collagen synthesis in relation to the donor age of human dermal fibroblasts. J. Invest. Dermatol. 103:228-232.
Quaglino Jr., D., Fornieri. C., Botti. B., Davidson, J. M., and Pasquali-Ronchetti, 1991, Opposing effects of ascorbate on collagen and elastin deposition in the neonatal rat aorta. Eur. J. Cell Biol. 54:18-26.
Quaglino, Jr., Zoia, O., Kennedy, R., and Davidson, J.M., 1989, Ascorbate affects collagen and elastin mRNAs in pig skin fibroblast cultures. Eur. J. Cell. Biol. Suppl 49,(28):45.
Rivers, J.M., Krook, L., and Cormier, S.A., 1970, Biochemical and histological study of guinea pig fetal and uterine tissue in ascorbic acid deficiency. J. Nutr. 100:217-227.
Robertson, W.B., and Schwartz, B., 1953, Ascorbic acid and the formation of collagen. J. Biol. Chem. 201:689-696.
Rosenbloom, J., and Cywinski, A., 1976, Inhibition of prolinehydroxylation does not inhibit secretion of tropoelastin by chick aorta cells, FEBS Lett. 65:246-250.
Rosenbloom, J., Harsch, M., and Jimenez. S., 1973, Hydroxyproline content determines the denaturation temperature of chick tendon collagen, Arch. Biochem. Biophys. 158:478-484.
Ross, R., and Benditt, E.P., 1962, Wound healing and collagen formation. II. Fine structure in experimental scurvy, J. Cell Biol. 12:533-551
Ross. R., and Benditt, E. P., 1965, Wound healing and collagen formation. Quantitative electron microscopy radiographic observations of proline-3H utilization by fibroblasts, J. Cell Biol. 27:83-106.
Russell, J. E., and Manske, P.R., 1991, Ascorbic acid requirement for optimal flexor tendon repair in vitro, J. Orthop. Res. 9:714-719.
Sandell, L. J., and Daniel, J.C., 1988, Effects of ascorbic acid on collagen mRNA levels in short-term chondrocyte cultures, Connect. Tissue Res. 17:11-22.
Schwartz, E., Bienkowski, R.S., Coltoff-Schiller. B., Goldfisher, S., and Blumenfeld, O.O., 1982, Changes in the components of extracellular matrix and in growth properties of cultured aortic smooth muscle cells upon ascorbate feeding. J. Cell Biol. 92:462-470.
Scott-Burden. T., Davies. P.J., and Gevers. W., 1979, Elastin biosynthesis by smooth muscle cell cultured under scorbutic conditions, Biochem. Biophys. Res. Commun. 91:739-746.
Senoo, H., and Hata, R., 1994, Extracellular matrix regulates and L-ascorbic acid 2-phosphate further modulates morphology, proliferation, and collagen synthesis of perisinusoidal stellate cells. Biochem. Biophys. Res. Commun. 200:999-1006.
Taylor, S.M., and Jones P.A., 1979, Multiple new phenotypes induced in 10T1/2- and 3T3 cells treated with 5-azacytidine, Cell 17:771-779.
Tuderman, L., Myllyla. R., and Kivirikko. K.I., 1977, Mechanism of the prolyl hydroxylase reaction. I. Role of co-substrates, Eur. J. Biochem. 80:341-348.
Uitto, J., Hoffmann, H.P.,and Prockop, D.J., 1976, Synthesis of elastin and procollagen by cells from embryonic aorta. Difference in the role of hydroxyproline and the effects of proline analogs on the secretion of the two proteins. Arch. Biochem. Biophys. 173:187-200.
Uitto, J., and Prockop, D.J., 1974, Hydroxylation of peptide-bound proline and lysine before and after chain completion of the polypeptide chains, Arch. Biochem. Biophys. 164:210-217.
Urry, D.W., Sugano, H., Prasad, K. U., Long, M.L., and Bhatnagar, R.S., 1979, Prolyl hydroxylation of the polypentapeptide model of elastin impairs fiber formation, Biochem. Biophys. Res. Commun. 90:194-198.
Volpin, D., and Pasquali-Ronchetti, I., 1977, The ultrastructure of high temperature coacervates of elastin, J. Ultrastruct. Res. 61:295-301.
Wegger, I., and Palludan, B., 1994, Vitamin C deficiency causes hematological and skeletal abnormalities during fetal development in swine, J. Nutr. 124:241-248.
Wilson, J.X., and Dixon, S.J., 1989, High-affinity sodium dependent uptake of ascorbic acid by rat osteoblasts, J. Membr. Biol. 11:83-91.
Wolbach, S.H., and Howe, P.K., 1926, Intercellular substances in experimental scorbutis, Arch. Pathol. Lab. Med. 1:1-24.
Wolfer, J.A., Farmer, C.J., Carroll, W.W., and Manshardt, D.0., 1947, Experimental study of wound healing in vitamin C depleted human subjects, Surg. Gynecol. Obstet. 84:1-15.
Yu, K., Kurata, T., and Arakawa, N., 1988, The behavior of L-ascorbic acid in the prolyl 4-hydroxylase reaction, Agric. Biol. Chem. 52:729-731.
Yue, B.Y.J.T., Higginbotham, E.J., and Chang, I.L., 1990, Ascorbic acid modulates the production of Fibronectin and laminin by cells from an eye tissue trabecular meshwork, Exp. Cell Res. 187:65-68.